38 how to cite fda drug label
FDA Label Search-Application Number FDA Label Search. FDA Home - Search by Application Number or Regulatory Citation: ... including the leading zero. For citations, type in "part" and at least a portion of the citation (e.g., part310)" Return to the FDA Label Search Page - - Links on this page: ... U.S. Food and Drug Administration. 10903 New Hampshire Avenue Silver Spring, MD ... FDA Label Search Search for Labels on DailyMed. The labels are also available on the National Library of Medicine's DailyMed. 4. web site. You can search for labels by drug name and link to the Library's information resources about marketed drugs.
How Do I Use Prescription Drug Labeling | FDA FDA-Approved Patient Labeling Patient labeling may be physically attached or provided separately from the USPI and contains information in lay language that can help patients use a drug safely and...

How to cite fda drug label
Q. How do I cite a drug package insert? - Ask the Research ... Reference Listed Drug, Reference Standard, Basis of Submission - FDA ... As we know, all drug products approved for safety or efficacy are cited in the Orange Book and are considered "listed" drugs. The FDA now is making a distinction between the designation of an RLD (the drug upon which an ANDA may be based) and a reference standard (the drug that FDA expects the firm to use for establishing bioequivalence). Labeling Information | Drug Products | FDA For prescription drug labeling resources (e.g., Prescribing Information, FDA-approved patient labeling, and carton and container labeling), please see the Prescription Drug Labeling Resources web page
How to cite fda drug label. › pmc › articlesTen Common Questions (and Their Answers) About Off-label Drug Use The term off-label drug use (OLDU) is used extensively in the medical literature, continuing medical education (CME) exercises, and the media. It is a polarizing term because it can be associated with great benefit or harm to patients.1 In addition, OLDU, along with allegations of pharmaceutical company promotion of OLDU, has been the cause of major lawsuits and historically large out-of-court ... Code of Federal Regulations Title 21 - Food and Drug Administration Listed drug status is evidenced by the drug product's identification in the current edition of FDA's "Approved Drug Products With Therapeutic Equivalence Evaluations" (the list) as an approved... Drug labeling, Information about Drug labeling - FAQs The FDA has been working to make drug labels better and to promote safer andmore effective use of drugs, particularly with regard to prescription drugs used in children. ... More than 70% of prescription drugs listedin the Physician's Desk Reference have no dosing information for treating children, and the lack of information is an obstacle to ... Reference Listed Drug, RLD, ANDA, Generic drug, FDA A Reference Listed Drug (RLD), as goes by its innate meaning, is an FDA approved drug product which can be referred to by a generic drug manufacturer while filing an Abbreviated New Drug Application (ANDA). An RLD is basically useful to establish bioequivalence of the product with that of an already approved one.
Referencing/Citing Drugs.com Subscribe to Drugs.com newsletters for the latest medication news, new drug approvals, alerts and updates. Drugs.com provides accurate and independent information on more than 24,000 prescription drugs, over-the-counter medicines and natural products. PDF Drug Name And NDC Reference Data Instructions CY2022 exclusively for a private label distributor, or drugs that are marketed solely as part of a kit or combination product or inner layer of a multi-level packaged product not marketed individually. • Drugs that do not have a marketed name. Contents of the Drug Name and NDC Reference Data . 1. The Drug Name and NDC Reference Data file: How to Read Drug Labels - WebMD 2 /7 Find this info at the top of the label on over-the-counter meds. It's the ingredient in the medicine that treats a symptom, along with the type of medication it is, like "antihistamine" or... en.wikipedia.org › wiki › Food_and_Drug_AdministrationFood and Drug Administration - Wikipedia The United States Food and Drug Administration (FDA or USFDA) is a federal agency of the Department of Health and Human Services.The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, dietary supplements, prescription and over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood ...
Referencing Approved Drug Products in ANDA Submissions Guidance for ... reference listed drug (RLD), a reference standard, and the basis of submission in an abbreviated new drug application (ANDA) submission. 2 Section 505(j) of the Federal Food, Drug, and Cosmetic Act... › scripts › cdrhCFR - Code of Federal Regulations Title 21 - Food and Drug ... Mar 29, 2022 · Label has the meaning set forth in section 201(k) of the Federal Food, Drug, and Cosmetic Act. Labeler means: (1) Any person who causes a label to be applied to a device with the intent that the device will be commercially distributed without any intended subsequent replacement or modification of the label; and Outdated Prescription Drug Labeling: How FDA-Approved Prescribing ... Although FDA-approved labeling can never be fully aligned with real-world clinical practice, steps should be taken to better align the two when high-quality data exist. Such steps, if taken, will assist patients and prescribers in discerning which uses of drugs are supported by the highest quality evidence. Keywords Prescription Drug Labeling Resources | FDA FDA's Prescription Drug Labeling Resources website provides over 150 labeling resources for the Prescribing Information, FDA-approved patient labeling, and/or carton and container labeling for ...
How to Read Over-the-Counter and Prescription Drug Labels The FDA requires manufacturers to write medication guides in easy-to-understand language for patients. You can find these medication guides on the drug manufacturer's website or on DailyMed. The blood thinner Coumadin, also known under the generic name warfarin, has a medication guide.
FDA Guidance on Differences Between RLD and Reference Standard for ANDA ... Ordinarily, the reference standard selected by FDA will be the RLD; however, that is not always so. If FDA has selected a reference standard for use in in vivo bioequivalence studies different from the RLD, then the ANDA applicant must compare its proposed product's labeling and formulation to that of the RLD and not to the reference standard.
Structured Product Labeling - Wikipedia Structured Product Labeling ( SPL) is a Health Level Seven International (HL7) standard which defines the content of human prescription drug labeling in an XML format. The "drug labeling" includes all published material accompanying a drug, such as the Prescribing Information which contains a great deal of detailed information about the drug.
Drug Information Portal - U.S. National Library of Medicine - Quick ... The NLM Drug Information Portal gives users a gateway to selected drug information from the National Library of Medicine and other key government agencies. More than 49,000 drugs can be searched. ... FDA CDER (drugs) FDA CBER (vaccines) Drug Enforcement Administration (DEA) Centers for Disease Control and Prevention (CDC - Vaccines)
Code of Federal Regulations Title 21 - Food and Drug Administration The requirements in this section apply only to prescription drug products described in § 201.56(b)(1) and must be implemented according to the schedule specified in § 201.56(c), except for the requirement in paragraph (c)(18) of this section to reprint any FDA-approved patient labeling at the end of prescription drug labeling or accompany the ...
› scripts › cdrhCFR - Code of Federal Regulations Title 21 - Food and Drug ... Mar 29, 2022 · (3) Wherever the name of the food appears on the label so conspicuously as to be easily seen under customary conditions of purchase, the labeling required by paragraph (b)(2) of this section shall immediately and conspicuously precede or follow such name, without intervening written, printed, or graphic matter. (c) Label declaration.
PDF Reference ID: 3326669 - Food and Drug Administration ProductComplaintsPP@hospira.com, or FDA at 1-800-FDA-1088 or . ... Reference ID: 3326669 . Pharmacokinetics FULL PRESCRIBING INFORMATION: CONTENTS* 1 . INDICATIONS AND USAGE ... 5.1 Drug Administration 5.2 Hypotension, Bradycardia, and Sinus Arrest 5.3 Transient Hypertension .
› zantac › recallZantac FDA Recall | Ranitidine Products & Cancer Concerns The FDA has determined that NDMA levels increase in some ranitidine products over time. The agency found NDMA levels were greater when more time had passed since the drug was manufactured. New FDA studies also confirmed significant increases in NDMA levels when samples were stored at higher temperatures.
FDA Prescribing Information for Professionals - Drugs.com FDA product labels provide Professional Information about drugs. This professional information presents product monographs approved by the US Food and Drug Administration (FDA) and compiled by drug manufacturers. Drugs.com reformats the style of these monographs, but the content is a duplicate of FDA-approved labeling.





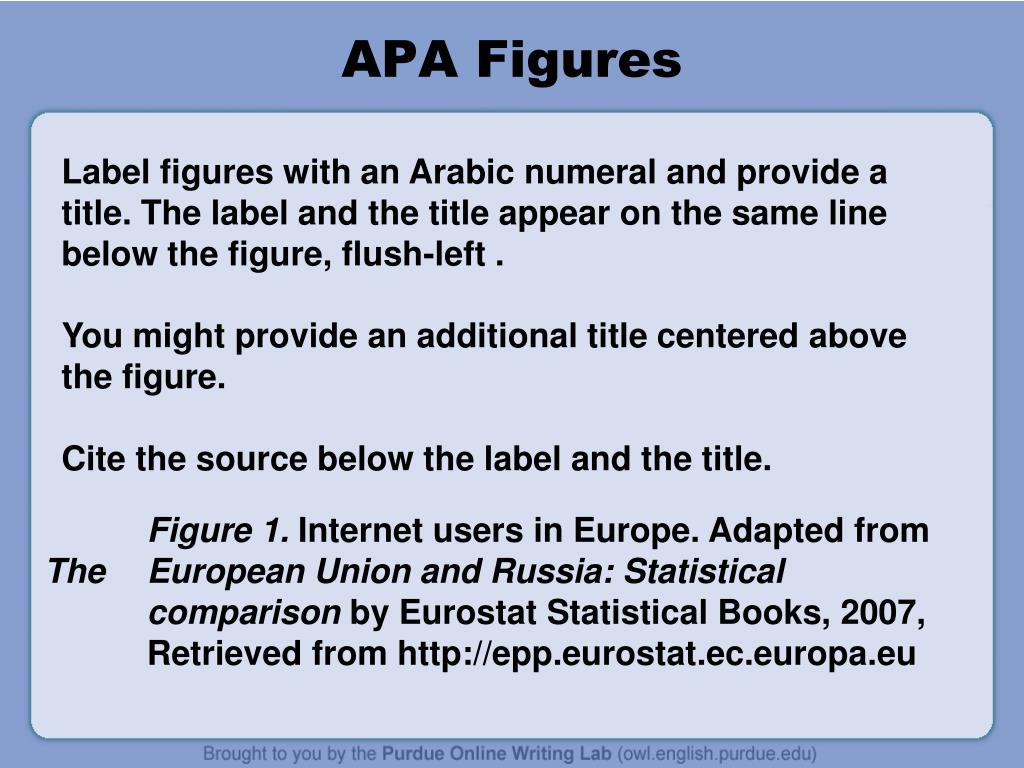

Post a Comment for "38 how to cite fda drug label"