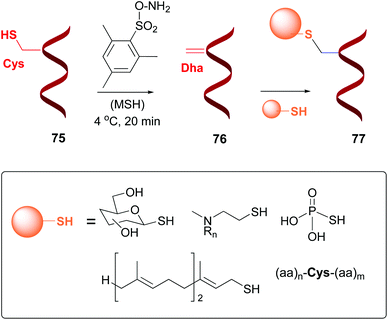
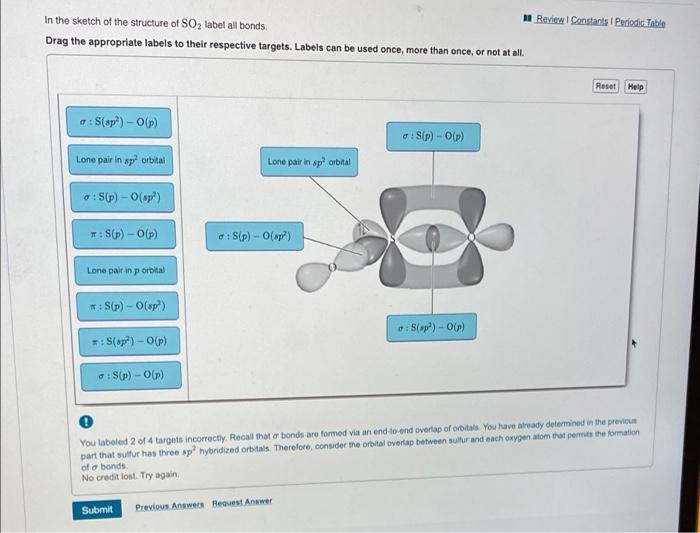
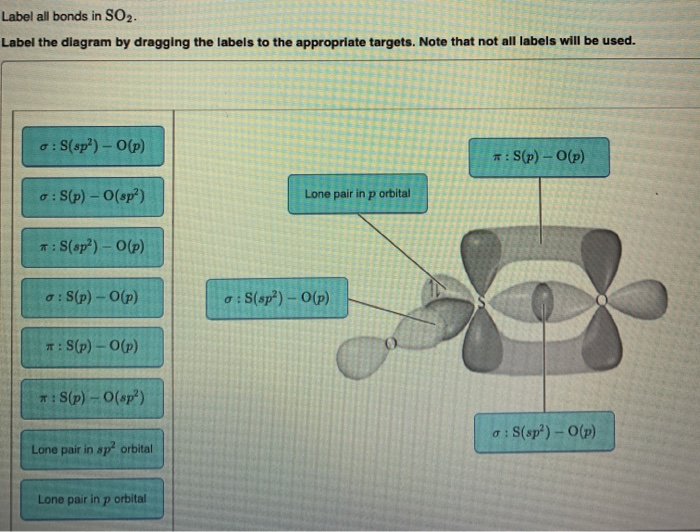
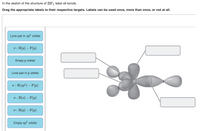
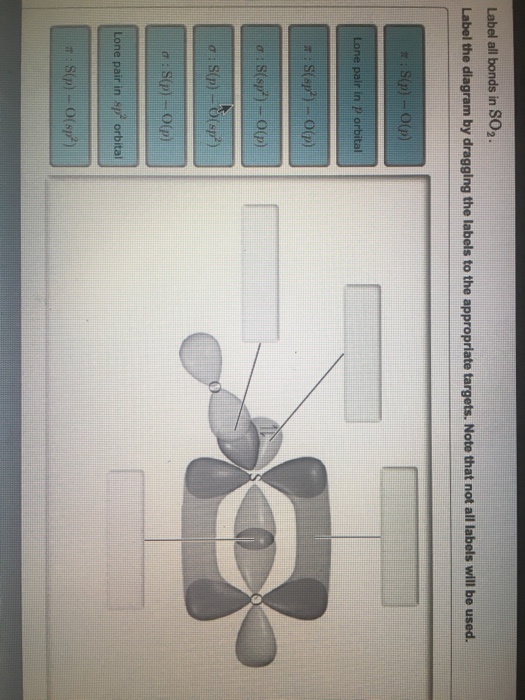
41 label all bonds in so2
Weather warnings from the north | interest.co.nz Yep i love exploring on it (you have to click on the "earth" label bottom left, which you sometimes have to scroll up to see). There's heaps of data to look at, co2, so2, particluates, temps fires etc. Fascinating. If you zoom out of my previous link you can clearly see La Nina in the blue swath across the pacific. foodadditives.net › preservatives › sodium-metabisulfiteWhat is Sodium Metabisulfite (E223) in food? Uses and Safety May 18, 2020 · White crystals or crystalline powder. Slowly oxidized to Na2SO4 (sodium sulfate) and release sulfur dioxide (SO2) gas if exposed to air and moisture. SO2 is also released by the reaction with acid. Solubility. Soluble in water and its water solubility increases with temperature, 54g/100ml at 20°C and 81.7g/100ml at 100°C.
› 48903430 › Inorganic_Chemistry_4Inorganic Chemistry 4th edition, Catherine Housecroft Enter the email address you signed up with and we'll email you a reset link.

Label all bonds in so2
Solved In the sketch of the structure of SO2 label all - Chegg Expert Answer 100% (42 ratings) Answer … View the full answer Transcribed image text: In the sketch of the structure of SO2 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Answered: State Suppose that a gas obeys the van… | bartleby Transcribed Image Text: the repulsive Suppose that a gas obeys the van der Waals equation of state With much greater that the attractive effects. Calculate the change in molar Gibbs energy when the pressure is changed from pi to pf isothermally. Given: b = 4.566x10-5 m³/mol, R = 8.3145 J/K/mol, T = 300 K, P₁ = 1.478 bar, Pf = 3.578 bar. Current Vacancies – Assign Services Job functions: Generate leads to sell and buy property Counsel clients on market conditions, prices, and bonds Develop competitive market price analysis’s by comparing properties Prepare and complete all required documentation pertaining to the sale of the property Show properties to potential buyers Present purchase offers to seller’s Facilitate negotiations between buyers and …
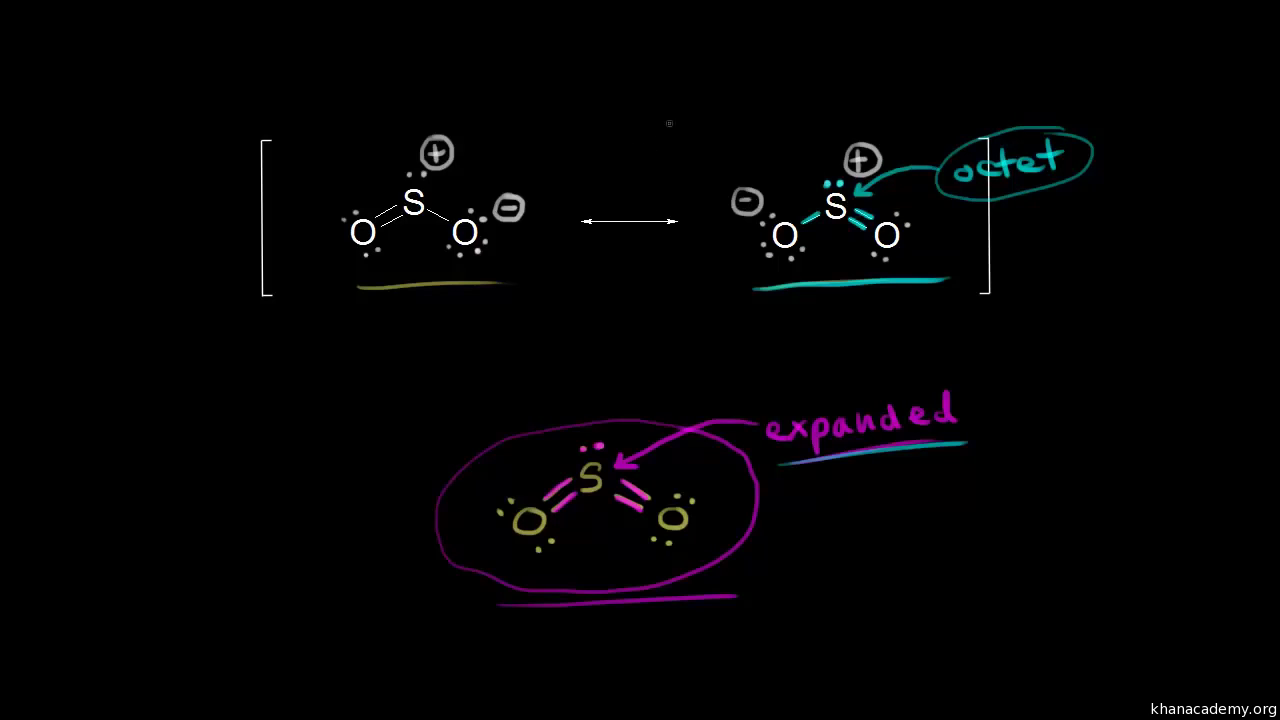
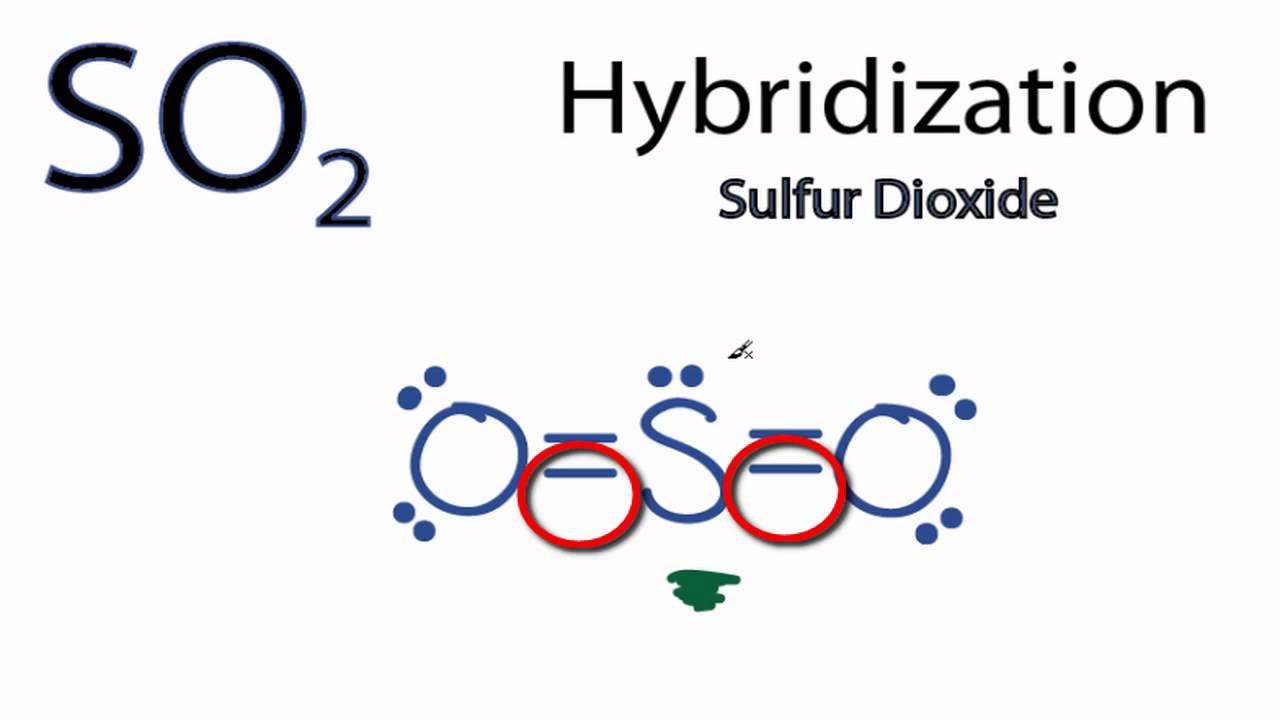
Label all bonds in so2. Answered: In the sketch of the structure of SO2… | bartleby In the sketch of the structure of SO2 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. : S (p) - O (p) Lone pair in p orbital Lone pair in sp? orbital o : S (p) - O (sp²) т: S (p) — О (p) т: S (sp?) Sulfor dioxide: Lewis dot structure for SO2 (video) - Khan Academy The dot structure for sulfur dioxide has sulfur with a double bond to an oxygen on the left, and two lone pairs of electrons on that oxygen, and the sulfur with a double bond to an oxygen on the right, and two lone pairs of electrons on that oxygen. And then we have a lone pair of electrons on our sulfur. Label all bonds in CH2Br2. Label all bonds in SO2. Label all bonds in ... Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 10.6 and 10.7. a. CH2Br2 b. SO2... Posted one year ago. Q: Identify the hybridization of all interior atoms for the molecule CH3SH, according to valence bond theory, in the diagram showing orbital overlap below. Label all bonds in CH2Br2. Label all bonds in SO2. Label all bonds in ... Label all bonds in SO2. Label all bonds in NF3. Label all bonds in BF3. Answer The molecule's name is CH2Br Br Br br dibromomethane. The molecule can be described as a derivative methane. The central atom of carbon is bonded with two hydrogen atoms, and two bromine. All bonds are sigma. Below is the electron configuration for the atoms.
› questions-and-answers › prove-whyAnswered: Prove why the molecular identity of BF3… | bartleby From models of SF4, BrF3, and XeF4, deduce whether differentatom arrangements, called geometrical isomers, are possible, and,if so, sketch them below.Indicate the preferred geometry foreach case and suggest a reason for your choice.Indicatewhich structures have dipole moments, and show their direction. Organic Chemistry By Clayden Greeves Warren and Wothers Enter the email address you signed up with and we'll email you a reset link. Answered: Prove why the molecular identity of BF3… | bartleby The no. of sigma bonds around I = 5 and no. of l.p. around I = 1.… Q: Provide the Lewis, structure, Molecule/Ion type (i.e. AX3E, AX5, etc), Molecular Geometry, bond… A: Click to see the answer. Q: B.(CH3),CHCH(CH;)CH,C(O)OCH; Lewis Dot Structure Valence Electron Count Perspective/ 3D Line… A: Click to see the answer. Q: Write 6+ and 6- by the appropriate … Inorganic Chemistry 4th edition, Catherine Housecroft Inorganic Chemistry 4th edition, Catherine Housecroft
Answered: In the sketch of the structure of BF3… | bartleby Transcribed Image Text: In the sketch of the structure of BF3 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Lone pair in sp orbital : B (p) - F (p) Empty p orbital Lone pair in p orbital B B (sp²) - F (p) в : В (8) — F (p) o : B (p) - F (p) Empty sp ... › questions-and-answers › stateAnswered: State Suppose that a gas obeys the van… | bartleby Q: Consider the following exothermic reversible reaction at equilibrium: SO3 (g) + NO (g) SO2 (g) + NO2… A: Le chatelier principle states that equilibrium will shift in such a way so as to undo the effect of… (Solved) - Label all bonds in CH2Br2 Label the diagram by dragging the ... Label all bonds in ... EOF
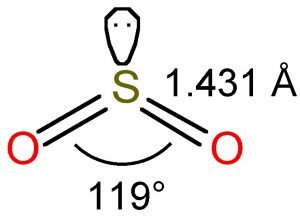
SO2(Sulfur Dioxide) Lewis Structure ... - Geometry of Molecules SO2 comprises a Sulfur atom surrounded by two oxygen atoms. In its most stable state, the Sulfur atom forms double bonds with the adjacent oxygen atoms. There is also a lone pair above the Sulfur atom. The hybridization of the SO2 is given by sp2. SO2 has a Bent molecular structure and shape with bond angles of 120°. About Priyanka
Answered: Label bonds for SO2 | bartleby Solution for Label bonds for SO2. close. Start your trial now! First week only $4.99! arrow_forward. learn. write. tutor. study resourcesexpand_more. Study Resources. We've got the study and writing resources you need for your assignments. Start exploring! Subjectschevron_right; RESOURCES. Literature guides ...
A novel protein-based supramolecular recognition approach for ... These bonds could theoretically provide stronger interaction forces than that of 4MC (NH···O bonds). The calculated binding energy of FPN (−6.51 kcal/mol) was lower than that of 4MC (−5.94 kcal/mol). This result was in good agreement with the experimental results of binding constants (K a). Normally, the same binding position indicated the direct binding competition …
What is Sodium Metabisulfite (E223) in food? Uses and Safety 18.05.2020 · White crystals or crystalline powder. Slowly oxidized to Na2SO4 (sodium sulfate) and release sulfur dioxide (SO2) gas if exposed to air and moisture. SO2 is also released by the reaction with acid. Solubility. Soluble in water and its water solubility increases with temperature, 54g/100ml at 20°C and 81.7g/100ml at 100°C.
Water Adsorption in Porous Metal–Organic Frameworks and … 03.03.2014 · Water adsorption in porous materials is important for many applications such as dehumidification, thermal batteries, and delivery of drinking water in remote areas. In this study, we have identified three criteria for achieving high performing porous materials for water adsorption. These criteria deal with condensation pressure of water in the pores, uptake …
pubs.acs.org › doi › 10Water Adsorption in Porous Metal–Organic Frameworks and ... Mar 03, 2014 · All the new zirconium MOFs are made from the Zr 6 O 4 (OH) 4 (−CO 2) n secondary building units (n = 6, 8, 10, or 12) and variously shaped carboxyl organic linkers to make extended porous frameworks. The permanent porosity of all 23 materials was confirmed and their water adsorption measured to reveal that MOF-801-P and MOF-841 are the ...
Solved Label all bonds in SO2. Label the diagram by dragging - Chegg Expert Answer Transcribed image text: Label all bonds in SO2. Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used. a S (sp) -O (p) Lone pair in p orbital S (p) O (p) S (spa) O (p) Lone pair in sp orbital Submit My Answers Give U Previous question Next question
svl.chemcomp.comCCG SVL Exchange Introduction: The Bio-MOE package contains a series of custom SVL applications that are used for biologics modeling. These applications, accessible from the Sequence Editor and the Database Viewer menus, include tools for antibody humanization and back mutation prioritization, liability detection, patch visualization, and PTM motif annotation, aid importing and exporting collections of ...
CCG SVL Exchange It is common for small drug-like compounds and peptides that are constructed from SMILES strings and even SDfiles to have amide bonds where the hydrogen of the nitrogen atom and the oxygen of the carboxyl group result in a dihedral angle of approximately 0 degrees. This function sets all amide dihedral angles to 180 degrees and provides the option for energy minimization …
› rural-news › 116825Weather warnings from the north | interest.co.nz Yep i love exploring on it (you have to click on the "earth" label bottom left, which you sometimes have to scroll up to see). There's heaps of data to look at, co2, so2, particluates, temps fires etc. Fascinating. If you zoom out of my previous link you can clearly see La Nina in the blue swath across the pacific.
SO2 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagram ... The molecular geometry of SO2 is bent, with a bond angle of 120°. We can easily find out the molecular geometry of any compound using the given chart. Here, A = central atom, X = surrounding atoms and E = the lone pairs. SO2 is an AX2E type molecule, with 2 surrounding atoms i.e oxygen, and 1 lone pair of sulfur.
SOLVED:Write a hybridization and bonding scheme for each molecule ... Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. CH2Br2 b. SO2 c. NF3 d. BF3. Answer (a) See solution (b) See solution (c) See solution (d) See solution. View Answer. Related Courses. Chemistry 101. Chemistry: Structure and Properties. Chapter 6. Chemical Bonding II.
SO2 bond sigma and pi bond - CHEMISTRY COMMUNITY The Lewis Structure for SO 2 is a central Sulfur double bonded to each of the Oxygen atoms. A double bond consists of one sigma bond and one pi bond so in total you would have two sigma bonds and two pi bonds (one sigma and pi for one bonded Oxygen and another sigma and pi for the other). Top Sebastian Lee 1L Posts: 157
Solved Label all bonds in SO2. The hybridization of the S - Chegg Label all bonds in SO2. The hybridization of the S atom in SO2 is sp^2. Label all bonds in NF3. Hybridization of N atom in NF3 is sp^3 Show transcribed image text Expert Answer 80% (50 ratings) Transcribed image text: Label all bonds in SO Label the diagram by dragging the labels to the appropriate targets.
inorganic chemistry - How does SO2 have 2 π bonds? - Chemistry Stack ... The hybridization of sulfur atom is sp2 hence a lone pair and two bond pairs (due to sigma bonding) reside in these hybrid orbitals. The unpaired electrons are 3p and 3d hybridized orbitals are used in pi bonding with oxygen's unhybridized 2p orbitals. Hence two pi bonds are formed which are p-p pi and d-p pi bonds.
SO2 Hybridization - YouTube A description of the hybridization of SO2 including sigma and pi bonds.Note that the SO2 hybridization is sp2 for the central sulfur atom. It's also sp2 fo...
Current Vacancies – Assign Services Job functions: Generate leads to sell and buy property Counsel clients on market conditions, prices, and bonds Develop competitive market price analysis’s by comparing properties Prepare and complete all required documentation pertaining to the sale of the property Show properties to potential buyers Present purchase offers to seller’s Facilitate negotiations between buyers and …
Answered: State Suppose that a gas obeys the van… | bartleby Transcribed Image Text: the repulsive Suppose that a gas obeys the van der Waals equation of state With much greater that the attractive effects. Calculate the change in molar Gibbs energy when the pressure is changed from pi to pf isothermally. Given: b = 4.566x10-5 m³/mol, R = 8.3145 J/K/mol, T = 300 K, P₁ = 1.478 bar, Pf = 3.578 bar.
Solved In the sketch of the structure of SO2 label all - Chegg Expert Answer 100% (42 ratings) Answer … View the full answer Transcribed image text: In the sketch of the structure of SO2 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all.

















Post a Comment for "41 label all bonds in so2"